FABERCO, BRINGING TO THE PHILIPPINES THE FIRST-EVER COVID-19 ORAL TREATMENT
Faberco Life Sciences Inc, an emerging Philippine-based pharmaceutical com-pany is one of the firsts to bring in COVID-19 drug Molnupiravir (Molnarz) to the country, making the Philippines the first country in South East Asia to have access to this first-ever COVID-19 oral treatment.
Molnupiravir is the first oral antiviral drug that will prevent mild to moderate cases of COVID-19 from progressing into severe disease that needs hospitali-zation.
Developed by Merck & Co, Inc, (MSD), it has given the manufacturing rights to eight top generic manufacturers in the world for low and middle-income coun-tries, including Faberco’s manufacturer, Aurobindo Pharma Ltd.
Faberco has currently made Molnupiravir (Molnarz) available in the Philippines through Compassionate Special Permit (CSP) issued by the Philippine Food and Drug Administration.
Though not yet available commercially, Faberco, has chosen RiteMed Philip-pines, Inc. to distribute Molnupiravir (Molnarz) to the private sector including hospitals, medical institutions, and treatment sites under compassionate use which allow institutions to procure and distribute products under study, or those awaiting approval for immediate use in emergency situations.
FABERCO EXPECTS PHILIPPINE FOOD AND DRUG ADMIN-ISTRATION EUA APPROVAL SOON
Faberco Life Sciences, Inc. (Faberco) expects the Philippine Food and Drug Administration (PFDA) to approve its Emergency Use Application (EUA) appli-cation for the use of Molnupiravir in the country after the oral treatment received EUA approval in the UK few weeks ago.
“We are anticipating the approval from the Philippine FDA in the coming weeks” said Faberco Life Sciences Inc. founding member and Chairman of the Board, Vinay Panemanglor.
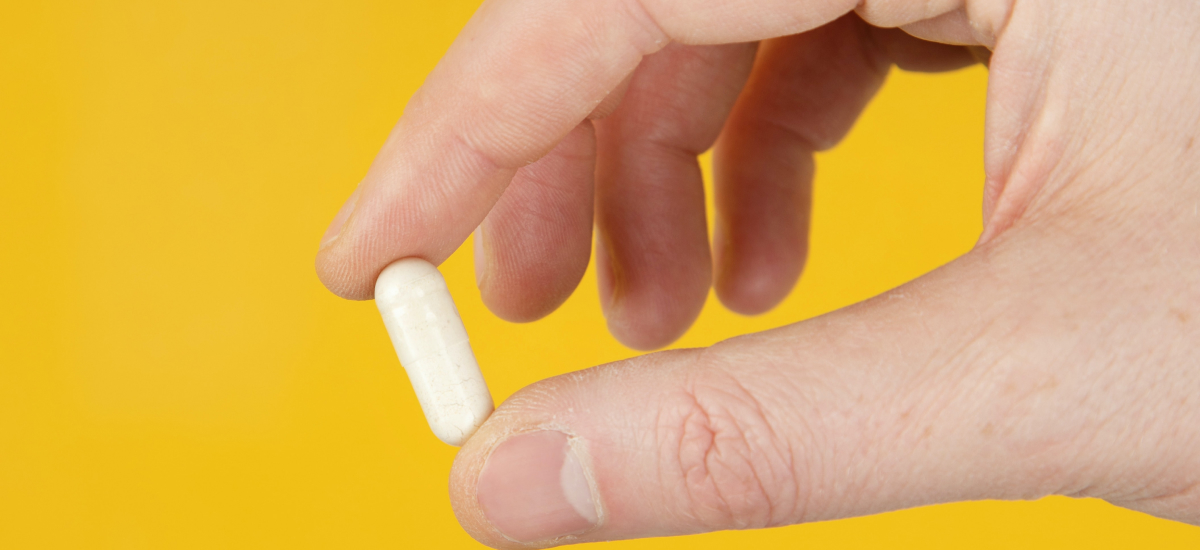
Molnupiravir, the first antiviral medication for COVID-19 is taken as a pill rather than given as an injection.
In clinical trials, the pill, originally developed to treat flu, cut the risk of hospitali-zation or death by 50% when given as a 5-day treatment regimen in patients with mild to moderate COVID-19. The drug needs to be given within five days of symptoms developing to be most effective.
Molnupiravir is the first COVID-19 drug that can be administered outside of a hospital setting, before the illness has progressed to a severe stage.
This milestone in the history of the country’s fight against COVID-19 would not have been possible if not for the support of the Philippine government. The company lauds and continuously supports the government’s efforts in providing COVID-19 vaccines and treatments, which remains to be the cornerstone in preventing severe disease and deaths due to COVID-19.
“Faberco Life Sciences, Inc commits to the Filipino people to make available and accessible this life-saving drug, bringing hope and giving our nation more reason to celebrate this Christmas and welcome the new year with more opti-mism and enthusiasm”, said Mr. Kishore N. Hemlani, the company’s founder and Vice-Chairman of the Board.
ABOUT FABERCO LIFE SCIENCES, INC.
Faberco Life Sciences Inc. (FLS), a Philippine biopharmaceutical organization, is focused on providing innovative, highly niche pharmaceutical products to both public and private health sectors. Faberco partners with reputable international manufacturers who target product platforms and therapeutic categories to treat and prevent diseases which are of public health concern.
For Inquiries:
Neil M. Maristela
Head of Public Relations
M: 09272344489
E: neil@faberco.ph